Grade: Pharmaceutical Grade
Factory Location: Guangzhou, China
Main Sales Markets: Asia,Middle East,Africa
Monthly Production Capacity: 1000kgs
Sample Provided: no
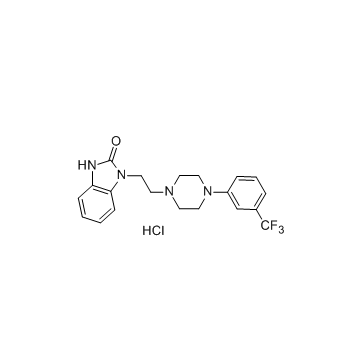
Name: Flibanserin HCl
MF: C20H22ClF3N4O
MW: 426.86
CAS:147359-76-0
Character: white powder
Purity(HPLC): 99.5%
Flibanserin (INN, USAN) (developmental code name BIMT-17; proposed trade names Girosa and Addyi) is a drug that is being studied as a non-hormonal treatment for pre-menopausal women with hypoactive sexual desire disorder (HSDD). Development by Boehringer Ingelheim was halted in October 2010 following a negative evaluation by the U.S. Food and Drug Administration. The rights to the drug were then transferred to Sprout Pharmaceuticals, which is continuing the drug development process. On June 4, 2015, the panel to the FDA recommended approval of the drug by 18–6.