Grade: Pharmaceutical Grade
Factory Location: Shandong
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East,Africa
Sample Provided: no
|
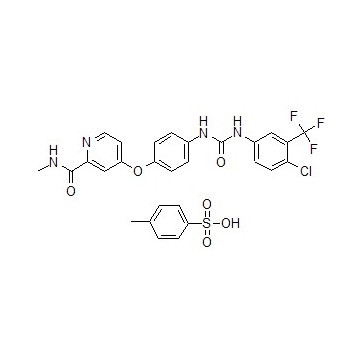
Sorafenib Tosylate with CAS 475207-59-1 is co-developed and co-marketed by Bayer and Onyx Pharmaceuticals as Nexavar.
Sorafenib Tosylate (CAS 475207-59-1) is approved in the US for the treatment of primary kidney cancer (advanced renal cell carcinoma), advanced primary liver cancer (hepatocellular carcinoma), and radioactive iodine resistant advanced thyroid carcinoma. |
The product above are not for sales where the patents are applicable and still valid.