Grade: Pharmaceutical Grade
Factory Location: SICHUAN
Main Sales Markets: Asia
Sample Provided: yes
Payment Terms: T/T
- Grade: Active Pharmaceutical Ingredient(API)
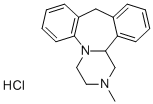
- Chemical Name: 1,2,3,4,10,14b-Hexahydro-2-methyl-dibenzo[c,f]pyrazino[1,2-a]azepine hydrochloride
- Molecular Formula:C18H20N2·HCl
- Molecular Weight:300.83
- Specification: Enterprise Standard established according to ChP/USP/EP
- Appearance: Powder
- Total impurities: not more than 0.5%
- Purity: not less than 99%
- Residual Solvents: fully comply with ICH Q3C
- Mutagenic impurities: fully comply with ICH M8
- Nitrosamine assessment: available
- Particle size: regular grade or milling/sieving according to customer’s requirement.
- Storage: Room temperature
- Production capacity: Commercial
- Standard Package: 1kg/bag, 5kg/bag, or according to the customer’s requirement