Grade: Pharmaceutical Grade
Factory Location: Wenzhou Zhejiang china china
Main Sales Markets: Asia
Sample Provided: no
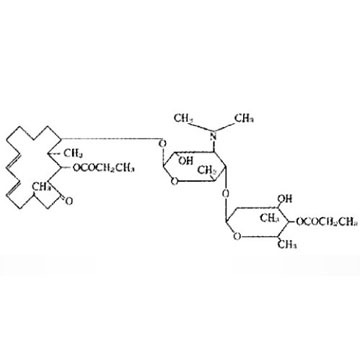
This product has strong antibacterial activity on gram-positive cocci, mycoplasma, gram-negativecocci, streptococcus pneumoniaeand pyogenic streptococcus, etc. Its lowest minimun inhibitory concentration on gram-positive cocci is 0.19-3.12mg/L, on Gram-negativecocci is 0.75-6.25mg/L, on mycoplasmais 0.0078mg/L.
Adaptation disease:
1. Pneumonia, lung fester disease, chronic bronchitis and other RTI.
2. Sore and furuncle, carbuncle, abscess and other skin and soft tissue infection.
3. Tonsillitis, sphagitis, acute otitis media and urinary tract infection, etc.
Adverse reactions and side effects are mainly: nausea, vomit, inappetence and diarrhea, etc.
Storage: Sealed and stored in dry place.
APIs packing: Kraft paper, lining with polythene film bag.
Tablet packing: Plastic packing. 12 tablets/board. 5 boards/box.
Specification: 0.1g/tablet.
Validity period: 2 years.
Approval number: ZWYZZ (1996)No. 129502