Grade: Pharmaceutical Grade
Factory Location: Shandong
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East,Africa
Monthly Production Capacity: 100kg
Contract Manufacturing: CRO,CMO
Packaging Information: 5kg/drum
Delivery Lead Time: 15days
Sample Provided: yes
Payment Terms: L/C
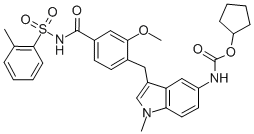
Vericiguat is a direct stimulator of soluble guanylate cyclase (sGC) used in the management of systolic heart failure to reduce mortality and hospitalizations. A key component of the NO-sGC-cGMP signaling pathway that helps to regulate the cardiovascular system, sGC enzymes are intracellular enzymes found in vascular smooth muscle cells (amongst other cell types) that catalyze the synthesis of cyclic guanosine monophosphate (cGMP) in response to activation by nitric oxide (NO). Cyclic GMP acts as a second messenger, activating a number of downstream signaling cascades that elicit a broad variety of effects, and these diverse cellular effects have implicated deficiencies in its production (primarily due to insufficient NO bioavailability) in the pathogenesis of various cardiovascular diseases. As a direct stimulator of sGC, vericiguat mitigates the need for a functional NO-sGC-cGMP axis and thereby helps to prevent the myocardial and vascular dysfunction associated with decreased sGC activity in heart failure. Vericiguat was approved by the FDA in January 2021 - developed by Merck under the brand name Verquvo - for use in certain patients with systolic heart failure. Although not the first sGC stimulator to be granted FDA approval ([riociguat] was approved in 2013 for use in pulmonary hypertension), vericiguat is unique amongst its peers in that modifications to its structure have dramatically decreased its susceptibility to oxidative metabolism, resulting in a relatively long half-life and allowing for once-daily dosing.