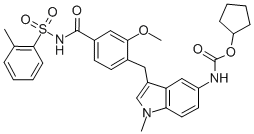
Grade: Pharmaceutical Grade
Factory Location: Shandong
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East,Africa
Monthly Production Capacity: 100kg
Contract Manufacturing: CRO,CMO
Packaging Information: 5kg/drum
Delivery Lead Time: 15days
Sample Provided: yes
Payment Terms: L/C
Mavacamten is a myosin inhibitor indicated for the treatment of adults with symptomatic New York Heart Association (NYHA) class II-III obstructive hypertrophic cardiomyopathy (HCM). It received initial US FDA approval in 2022, and it is one of the first myosin inhibitors to be used in humans. Mavacamten was also approved by Health Canada in October 2022 and by EMA in July 2023 for the same indication.