Grade: Pharmaceutical Grade
Factory Location: CHENLEI Science Park, 1 Qunxing Road,Suzhou Industrial Park, P.R. China
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East
Monthly Production Capacity: 100kg
Packaging Information: 5kg/tin; as per your requirements: Aluminum foil bag
Delivery Lead Time: 1 week
Sample Provided: yes
Payment Terms: L/C
API drug development and manufacturing services
Scientific expertise that speeds your journey
The successful development and manufacture of small molecule active pharmaceutical ingredients (API) rests on speed, efficiency, and quality across your journey. We offer scientific expertise, world-class technology, and a comprehensive range of solutions to help you reach your clinical milestones quickly and accelerate your path to commercialization.
Partner with us to seamlessly align the development and manufacture of your small molecule API and finished drug product. We use an integrated one-team approach to deliver the power of our full network. The Pharmaserve Quick to Clinic small molecule program is designed to accelerate your early development program for small molecules. We can also streamline your total drug substance and drug product development, demand planning, clinical trial, and commercial supply into a single customized solution with our Pharmaserve Quick to Care program.
From early process optimization and scale-up to commercial production, we provide key services across the lifecycle to get your project to market. Our focus is always on your molecule—whether you require early-phase material or late-phase and commercial supply, we have the capabilities, expertise, and experience to meet your API needs.
When the device of the invention is used to produce Tirzepatide API, the overall reaction time is reduced by 20% ‑ 30%; At the same time, the production cost of the whole process is reduced, making the cost reduced by about 10%, which is suitable for promotion and use, and promoting the further research and development of Tirzepatide.
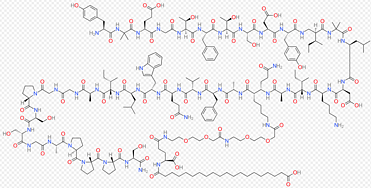
Purification of Pharmaserve Tirzepatide API
There are many impurities in the crude product of solid phase synthesis of Tirzepatide that affect the purity and yield of the sample. At present, there are many studies on the purification of Tirzepatide, and the purity can reach more than 99%. However, the solubility of the finished product obtained by the existing purification methods is not good, and other means are needed to improve the solubility of the finished product in the subsequent research and application process. Pharmaserve uses the latest peptide purification technology. The purity of the finished product reaches 99%, and the single impurity is less than 0.2%. At the same time, the solubility of the finished product is good, and the sample recovery rate can reach 100%. This process is stable and controllable, suitable for industrial production, and it can effectively reduce your scientific research or industrial needs.
Tirzepatide Approved for Weight Management in China
Recently, Eli Lilly’s Tirzepatide injection solution received approval from the National Medical Products Administration (NMPA) in China for long-term weight management in adults with obesity or overweight patients with at least one weight-related comorbidity. This approval marks the second indication for Tirzepatide in China, following its initial approval in May 2022 for blood sugar control in adults with type 2 diabetes.
As a glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist, Tirzepatide works by reducing food intake and regulating appetite to lower body weight and fat mass. In clinical trials, Tirzepatide demonstrated significant weight loss effects, with the 15mg dose group achieving an average weight loss of 20.9% over 72 weeks, the 10mg dose group averaging a 19.5% weight loss, and the 5mg dose group averaging a 15% weight loss.
Furthermore, the safety of Tirzepatide was also verified in clinical trials, with most adverse events being mild to moderate and occurring mainly during the dose-escalation period. This approval provides a new treatment option for patients with obesity and overweight, and also adds a new weapon for Eli Lilly in the GLP-1 market competition in China.
Globally, the GLP-1 drug market is rapidly expanding, with an expected scale of 40.7 billion US dollars by 2030. The launch of Tirzepatide will undoubtedly bring huge commercial opportunities for Eli Lilly and may change the existing market competition pattern.
Tirzepatide and Weight Loss Treatment: Paving New Ground
In the field of weight loss treatment, tirzepatide has shown tremendous potential. Clinical trial data indicate that tirzepatide is significantly effective in weight reduction, approaching the effects of common bariatric surgery. This discovery offers a new treatment option for obese patients, especially those who struggle with traditional weight loss methods.
The weight loss effects of tirzepatide are closely related to its unique mechanism of action. As a GIP/GLP-1 receptor agonist, tirzepatide not only lowers blood sugar but also increases satiety, reducing food intake and thus aiding in weight reduction. Additionally, the weight loss effects of tirzepatide are evident at different dosages, providing more treatment options for doctors and patients.
Although tirzepatide shows great potential in weight loss treatment, its safety and long-term efficacy still require further research. Currently, the weight loss indication for tirzepatide has been approved in some regions, and it is expected that more countries and regions will include it as an option for weight loss treatment in the future. As research on tirzepatide continues, we can expect more data and experience on its efficacy and safety in weight loss treatment.