Grade: Pharmaceutical Grade
Factory Location: shan dong
Main Sales Markets: Asia
Monthly Production Capacity: 50kg
Contract Manufacturing: CRO,CMO
Packaging Information: 1-5kg/Cardboard bucket
Delivery Lead Time: 3-5 Day
Sample Provided: no
Payment Terms: LC/DP/OA/TT
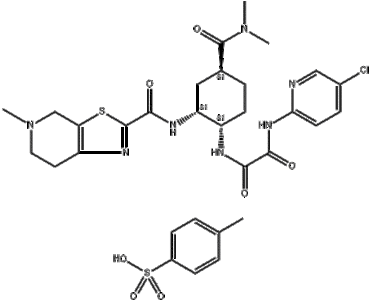
On January 8, 2015, the United States FDA approved the anticoagulant drug Edoxaban from the Japanese pharmaceutical company DaiichiSankyo under the trade name Savaysa. Idoxaban is an oral Chemicalbook selective coagulation factor Xa inhibitor for the prevention and treatment of deep vein thrombosis and pulmonary embolism, used to reduce the risk of stroke and systemic embolism events in patients with NVAF, currently available in 60mg, 30mg, and 15mg.