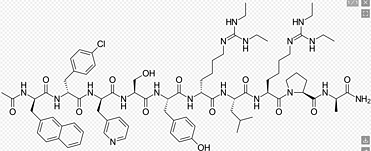
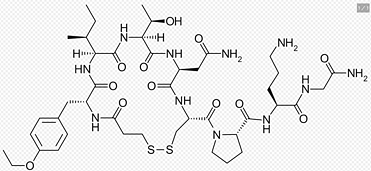
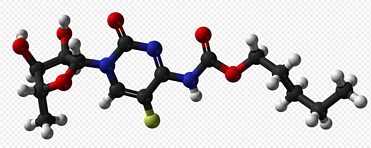
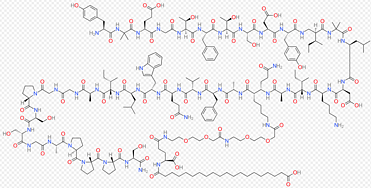
Grade: Pharmaceutical Grade
Factory Location: China
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Middle East
Monthly Production Capacity: 150kg
Packaging Information: 5kg/tin; Aluminum foil bag
Delivery Lead Time: In stock
Sample Provided: no
Payment Terms: L/C
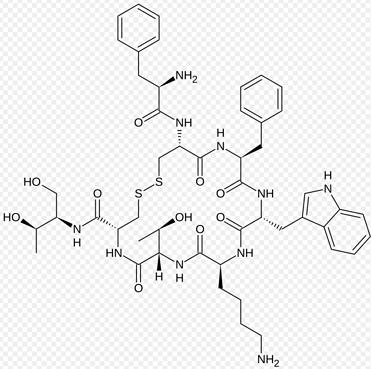
What is Ganirelix Acetate?
GANIRELIX is used to regulate hormone responses in women undergoing treatment for female infertility.
Indication
Ganirelix Acetate Injection is indicated for the inhibition of premature luteinizing hormone (LH) surges in women undergoing controlled ovarian hyperstimulation.
Mechanism of Action:
Ganirelix Acetate acts by competitively blocking the GnRH receptors on the pituitary gonadotroph and the subsequent transduction pathway Artistic rendering of competitive blocking of single GnRH receptor on the pituitary gonadotroph. Ganirelix Acetate acts by competitively blocking the gonadotropin-releasing hormone (GnRH) receptors on the pituitary gonadotroph and subsequent transduction pathway. It induces a rapid, reversible suppression of gonadotropin secretion. The suppression of pituitary luteinizing hormone (LH) secretion by Ganirelix Acetate is more pronounced than that of follicle-stimulating hormone (FSH). An initial release of endogenous gonadotropins has not been detected with Ganirelix Acetate, which is consistent with an antagonist effect. Upon discontinuation of Ganirelix Acetate, pituitary LH and FSH levels are fully recovered within 48 hours. Ganirelix Acetate must be present at the receptor site continuously to displace native GnRH.
Pharmaserve’s Technology of Synthesis
Ganirelix Acetate API Solid phase reactor is required in the production of Ganirelix Acetate API. Pharmaserve adopts a new type of special solid phase reactor for Ganirelix Acetate, including reactor shell, air inlet pipe, air outlet pipe, gas distribution component and solid phase catalytic component. In the production process of Ganirelix Acetate product itself, the equipment of the invention is suitable for solid phase catalytic reaction, which improves the reaction effect, shortens the reaction time, and is suitable for the production of Ganirelix Acetate, making the final product more pure.
When the device of the invention is used to produce Ganirelix Acetate API, the overall reaction time is reduced by 20% ‑ 30%; At the same time, the production cost of the whole process is reduced, making the cost reduced by about 10%, which is suitable for promotion and use, and promoting the further research and development of Ganirelix Acetate.
Purification of Pharmaserve Ganirelix Acetate API
There are many impurities in the crude product of solid phase synthesis of Ganirelix Acetate that affect the purity and yield of the sample. At present, there are many studies on the purification of Ganirelix Acetate, and the purity can reach more than 99%. However, the solubility of the finished product obtained by the existing purification methods is not good, and other means are needed to improve the solubility of the finished product in the subsequent research and application process. Pharmaserve uses the latest peptide purification technology. The purity of the finished product reaches 99%, and the single impurity is less than 0.2%. At the same time, the solubility of the finished product is good, and the sample recovery rate can reach 100%. This process is stable and controllable, suitable for industrial production, and it can effectively reduce your scientific research or industrial needs.
Infertility Key Facts