Grade: Pharmaceutical Grade
Factory Location: Xiamen, Fujian
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East
Monthly Production Capacity: 1000kg
Packaging Information: 25kg/drum 1kg/bottle
Delivery Lead Time: 7 days after payment
Sample Provided: yes
Payment Terms: L/L
|
Product name |
Fondaparinux sodium |
|
CAS No. |
114870-03-0 |
|
Molecular Formula |
C31H43N3O49S8*10Na |
|
Molecular Weight |
1728.1 |
|
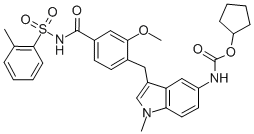
Molecular Structure |
|
|
Quality Standard |
95%-103% |
|
Appearance |
White or off- white powder |
|
COA |
|
ANALYSIS |
SPECIFICATION |
RESULTS |
|
Appearance |
White or off-white powder |
White powder |
|
Solubility |
Very soluble in water |
Very soluble in water |
|
Specific rotation |
+50° ~+65° |
58.5° |
|
Identification |
HPLC: The sample main peak Retention time shall matches with standard main peak Retention time in the Assay Determination Chromatogram. |
Conforms |
|
IR: The infrared absorption spectrum of the sample shall be concordant with that of Cannabidiol standard spectrum. |
Conforms |
|
|
Na: The sample main peak Retention time shall matches with standard main peak Retention time in the Sodium Determination Chromatogram. |
Conforms |
|
|
pH |
6.0-8.0 |
7.4 |
|
Appearance of solution |
The solution is clear and colourless |
Conforms |
|
Sodium |
11.5%-15.0% |
13.2% |
|
Related substances |
Impurity A ≤ 0.3% |
0.04% |
|
Impurity B ≤ 0.3% |
ND |
|
|
Any unspecified Impurity ≤ 0.3% |
0.03% |
|
|
Total Impurity ≤ 2.0% |
0.07% |
|
|
Free sulfate and chloride |
Free sulfate ≤ 0.30% Chloride ≤ 1.0% |
0.09% 0.05% |
|
Residual solvents |
Pyridine ≤ 50 ppm Toluene ≤ 200 ppm |
ND ND |
|
Water |
< 15% |
9.2% |
|
Palladium |
< 1ppm |
<0.05ppm |
|
Heavy metals |
< 20 ppm |
Conforms |
|
Microbial limit |
Total Aerobic Bacteria Count < 100cfu/g Total Mold & Yeast Count< 100cfu/g |
< 10cfu/g < 10cfu/g |
|
Bacterial Endotoxins |
Not more than 3.3EU/mg |
Conforms |
|
Assay |
95.0%-103.0% (on anhydrous basis&solvent free basis) |
99.5% |
|
Conclusion |
The product meets specification |
|
|
Usage |
What is Fondaparinux Sodium?
Fondaparinux Sodiumis a generic drug used to prevent a type of blood clot called deep vein thrombosis (DVT), which can lead to blood clots in the lungs (pulmonary embolism). Fondaparinux Sodium is used together with another anticoagulant to stop the blood clotting substance in the in the blood. This reduces the risk of heart attack, stroke, or breathing problems.
Some of the advantages with fondaparinux are its chemical nature of synthesis, minimal risk of contamination, 100% absolute bioavailability subcutaneously, instant onset of action, a long half-life, direct renal excretion, fewer adverse reactions when compared with direct oral anticoagulants.