Grade: Pharmaceutical Grade
Factory Location: Anqing,Anhui province
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East,Africa
Monthly Production Capacity: 500kgs
Packaging Information: 25kgs/drum
Delivery Lead Time: Prompt shipment
Sample Provided: yes
Payment Terms: T/T
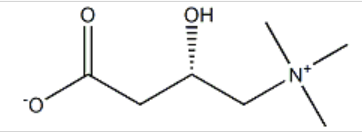
A 45-year-old woman with multiple sclerosis developed chronic fatigue syndrome associated with a carnitine deficiency, which was attributed to her immunosuppressive therapy with azathioprine. Supplementation with levocarnitine 6 g/day [ route not stated ] was initiated, and from the first day of treatment, she reported a bad, permanent "fishy" body odour that was extremely unpleasant. Associating the development of the odour with the start of levocarnitine, she discontinued the treatment and the odour resolved within 24 hours. The odour reappeared after an attempt to restart levocarnitine for 2 days. Treatment with withdrawn and the odour disappeared without recurrence. Author Comment The time of onset and regression after stopping levocarnitine in addition to the recurrence after reintroduction all support the role of the latter in the occurrence of this effect. Key words Trimethylaminuria - Levocarnitine - adverse reactions - drug-induced