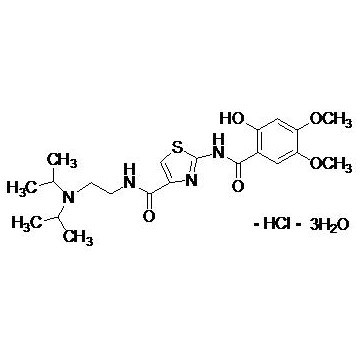
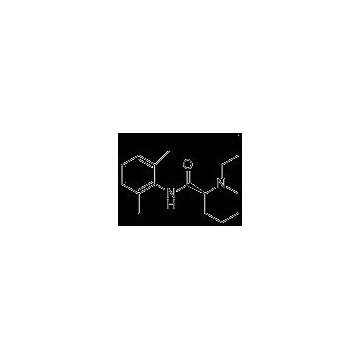
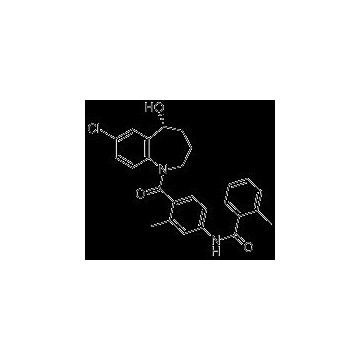
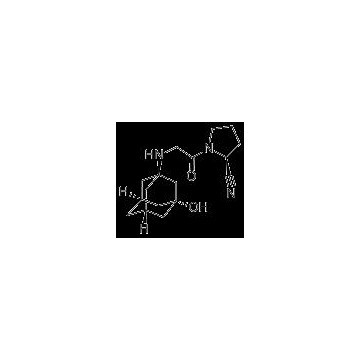
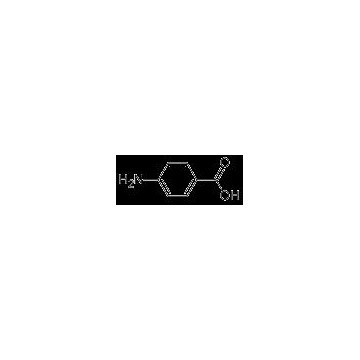
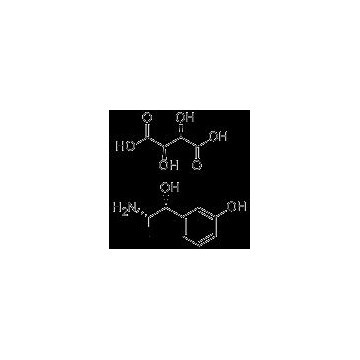
Grade: Pharmaceutical Grade
Factory Location: Changzhou, Jiangsu
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East
Sample Provided: no
Changzhou Sunlight Pharmaceutical Co., Ltd. (SunlightPharma) is located in Benniu Town, Changzhou City, Jiangsu Province, China. It is close to the Beijing-Shanghai High-Speed Railway, Shanghai-Nanjing Expressway, and Changzhou Airport via convenient transportation. SunlightPharmawas founded in 1990.Its campus covers a total area of 369,580㎡. Both of its Changzhou API andIntermediateProduction Site and Changzhou Pharmaceutical Formulation Production Site are located in BenniuBiomedical Industry Park. ItsJiangsu Sunlight Pharmaceutical and Chemical Material Co., Ltd. is located in the National Development Zone-Jiangsu CoastalIndustrial Park.
SunlightPharma is a national High-Tech enterprise. It is a Jiangsu AAA Credit Enterprised, Outstanding Scientific and technological Enterprised company.It is also recognized as a progressive and environmentalyfriendly company. The company consists of the R&D Division, the Manufacturing Division and the Sales Division. It has already been certified by the ISO 9001:2008 Quality Management System,the ISO 14001:2004 Environmental Management System, and OHSAS 18001 Occupational Health and Safety Management System.SunlightPharma hasalready obtained the Chinese Pharmaceutical Manufacturing Permit which allows the company to produce pharmaceutical chemical intermediates, APIs and finished products. With 80% of our products being exported to international markets such as the USA, Europe, and Southeast Asia, SunlightPharma has built a steady cooperative relationship with hundreds of clients, including top 500 enterprises. We have already passed the audits by EDQM, USFDA and Chinese GMP for our APIs, and have gotten boththe COS certificate and EUGMP certificate.
SunlightPharmacuttently has over 500 employees, including some foreign experts, and graduates from European, American, Russian, Japanese, Korean and Indian universities.Our R&D center has an excellent R&D team consisting of PhDs, professorsand senior engineers. We have already obtained 59 patents in China. We have the leading technology of Catalytic Hydrogenations, Reductive Alkylations, Chiral Resolutions, High-Pressure Reactions, and Cryogenic Reactions in China.Our Formulation R&D Laboratory is equipped with advanced instruments, which can fulfill the R&D tasks for both general solid dosage forms and sustained-and-controlled release solid dosage forms.
SunlightPharma owns modern manufacturing workshops for API and Oral Solid Formulations, which are designed and constructed in accordance with the national safety standards and GMP requirements. The API Workshop is fully equipped with reactors with volume ranging from 100L to 6000L, temperature ranging from-78°C to 300°C, and the pressure up to 12.5MPa. The Oral Solid FormulatonWorkshop is equipped with granulation linkage lines, fully automatic clean air conditioning system, and a purified water preparation system. It can produce tablets, capsules, and granules simultaneously to meet customers’ demands for custom-manufactured products.
We have already imported and installed advanced analytical instruments from America and Japan. These instruments include LC-MS, GC-MS, GC, HPLC, UPLC, FT-IR, UV/VIS, AA, automatic KFmeters as well as automatic titrators.
Our modern warehouse of raw materials and finished products is managed under the ERP management system. We also havea dedicated warehouse controlling the temperature and humidity at storage. SunlightPharma has the highest commitments for environmental protection.We have already built two 8500m³ waste water treatment and purifying sites, so as to guarantee the company’s sustainable development and provide stable service to our customers.
SunlightPharma committed ourselves to "Create benefits for customers,Create prosperity for society,Create futures for employees,Create value for shareholders" and "Promote health for mankind" with the mind set of “ Quality, Serive, and Reputation First”. We will keep continuing to provide the highest quality products and service for our clients and society.