Grade: Pharmaceutical Grade
Factory Location: Hai dian Beijing china china
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Asia,Africa
Contract Manufacturing: CRO,CMO
Sample Provided: yes
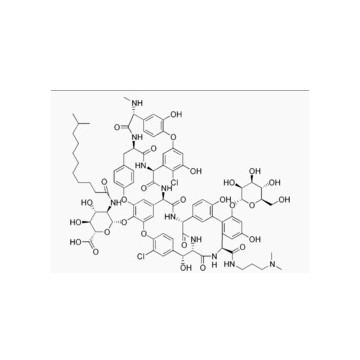
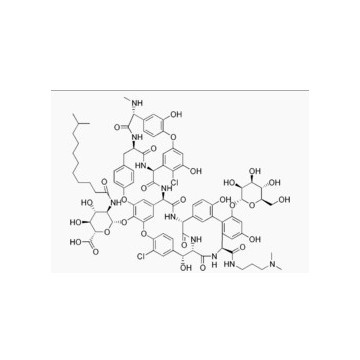
171500-79-1 lipoglycopeptide antibiotic Product Dalbavancin
Specification:
Dalbavancin (INN, trade name Zeven) is a novel second-generation lipoglycopeptide antibiotic. It belongs to the same class as vancomycin, the most widely-used and one of the few treatments available to patients infected withmethicillin-resistant Staphylococcus aureus (MRSA).
Dalbavancin (BI397) is a novel semisynthetic lipoglycopeptide that was designed to improve upon the naturalglycopeptides currently available, vancomycin and teicoplanin.
It possesses in vitro activity against a variety of Gram-positive pathogens including MRSA and MRSE. It is a once-weekly, two-dose antibiotic that Pfizer acquired when it bought Vicuron Pharmaceuticals in 2005.
Dalbavancin has undergone a phase III clinical trial for adults with complicated skin infections, but in Dec 2007 the FDA said more data was needed before approval.On September 9, 2008, Pfizer announced that it will withdraw all marketing applications in order to conduct another Phase 3 clinical trial. Durata Therapeutics acquired the rights to dalbavancin in December 2009 and has initiated two new Phase III clinical trials for treatment of acute bacterial skin and skin structure infections.Preliminary results in Dec 2012 looked good.