Grade: Pharmaceutical Grade
Factory Location: Shandong
Main Sales Markets: North America,Central/South America,Western Europe,Eastern Europe,Australasia,Asia,Middle East,Africa
Monthly Production Capacity: 100kg
Contract Manufacturing: CRO,CMO
Packaging Information: 5kg/drum
Delivery Lead Time: 15days
Sample Provided: yes
Payment Terms: L/C
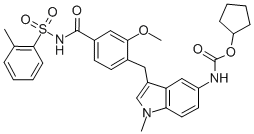
Edarbi (azilsartan medoxomil), a prodrug, is hydrolyzed to azilsartan in the gastrointestinal tract during absorption. Azilsartan is a selective AT1 subtype angiotensin II receptor antagonist. The drug substance used in the drug product formulation is the potassium salt of azilsartan medoxomil, also known by the US accepted name of azilsartan kamedoxomil and is chemically described as (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate monopotassium salt.