- Information for AAV2 (Catalog # 50459-AAV2)
Purpose
Ready-to-use AAV2 particles produced from pAAV-hSyn-DIO-mCherry (#50459). In addition to the viral particles, you will also receive purified pAAV-hSyn-DIO-mCherry plasmid DNA.
hSyn-driven, Cre-dependent mCherry-expression control. These AAV preparations are suitable purity for injection into animals.Delivery
- Volume100 µL
- Titer≥ 4×10¹² vg/mL
- Pricing$375 USD for preparation of 100 µL virus + $30 USD for plasmid.
- StorageStore at -80℃. Thaw just before use and keep on ice.
- ShipmentViral particles are shipped frozen on dry ice. Plasmid DNA (≥ 200ng) will also be included in the shipment.
Viral Production & Use
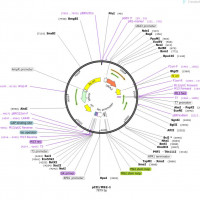
- Packaging Plasmidsencode adenoviral helper sequences and AAV rep gene, AAV2 cap gene
- BufferPBS + 0.001% Poloxamer 188
- SerotypeAAV2
- PurificationCsCl gradient ultracentrifugation
- Reporter GenemCherry (Cre-dependent)
Biosafety
Requestor is responsible for compliance with their institution's biosafety regulations. Lentivirus is generally considered BSL-2. AAV is generally considered BSL-1, but may require BSL-2 handling depending on the insert.
Addgene Comments
Using FLEX vectors in vivo: LoxP sites in FLEX plasmids are known to recombine during DNA amplification and viral vector production, which may result in a minority of Cre-activated (i.e., "flipped") viral vectors. Addgene has measured this occurs in 0.1-0.8% of viral particles in our typical production protocol. This can lead to a small number of cells exhibiting Cre-independent transgene expression in vivo. To address this, it is necessary to optimize the injection volume and viral titer to find the optimal AAV dosage required for Cre-dependent transgene expression and function in vivo. This may include reducing the viral particle dosage in order to reduce the likelihood of Cre-independent expression.




Addgene原装进口AAV病毒50459-AAV2
-
Favorite
-
Price
-
-
Negotiable
-
北京中源合聚生物科技有限公司
010-84415766
- Brand Addgene
Other Products from This Company
-
面议
-
面议
-
面议
-
面议
-
面议
-
面议
- Region:
- Main Business:试剂,仪器,耗材,细胞,质粒,进出口服务
The information on this page regarding the "Addgene原装进口AAV病毒50459-AAV2" product is provided by 北京中源合聚生物科技有限公司. If you would like to know more about the price, model, and manufacturer of "Addgene原装进口AAV病毒50459-AAV2", please contact the supplier or leave a message.
| Inquiries | N/A |
| Shipping | |
| Brand | Addgene |
| Expires | Long-term |
| Last Updated | 2025-08-12 16:02 |
Addgene原装进口AAV病毒50459-AAV2
| Company Name | 北京中源合聚生物科技有限公司 |
|---|---|
| Location | |
| Business Scope | 试剂,仪器,耗材,细胞,质粒,进出口服务 |
Contact Information
Similar Products Recommended
-
面议
-
面议
-
面议
-
面议
-
面议
- Disclaimer:
- All product information on this page, including prices, is provided by the supplier. The supplier is solely responsible for the authenticity, accuracy, and legality of the information. Medicine Platform offers no guarantees and assumes no liability for any resulting transactions or disputes.
- Reminder:
- We recommend you contact the supplier to confirm the final price and request samples to verify quality. Be cautious with unusually low prices, which may indicate fraud. Please verify all details before proceeding with a transaction.